3.2.S.2.2 - Description of Manufacturing Process and Process Controls
Section 3.2.S.2.2 should be provided in both the applicant's and restricted parts of the DIFA. The information should comprise all manufacturing steps from the introduction of the starting materials, which must comply with GMP. The open part should contain the synthetic scheme and simplified description of the manufacturing process.
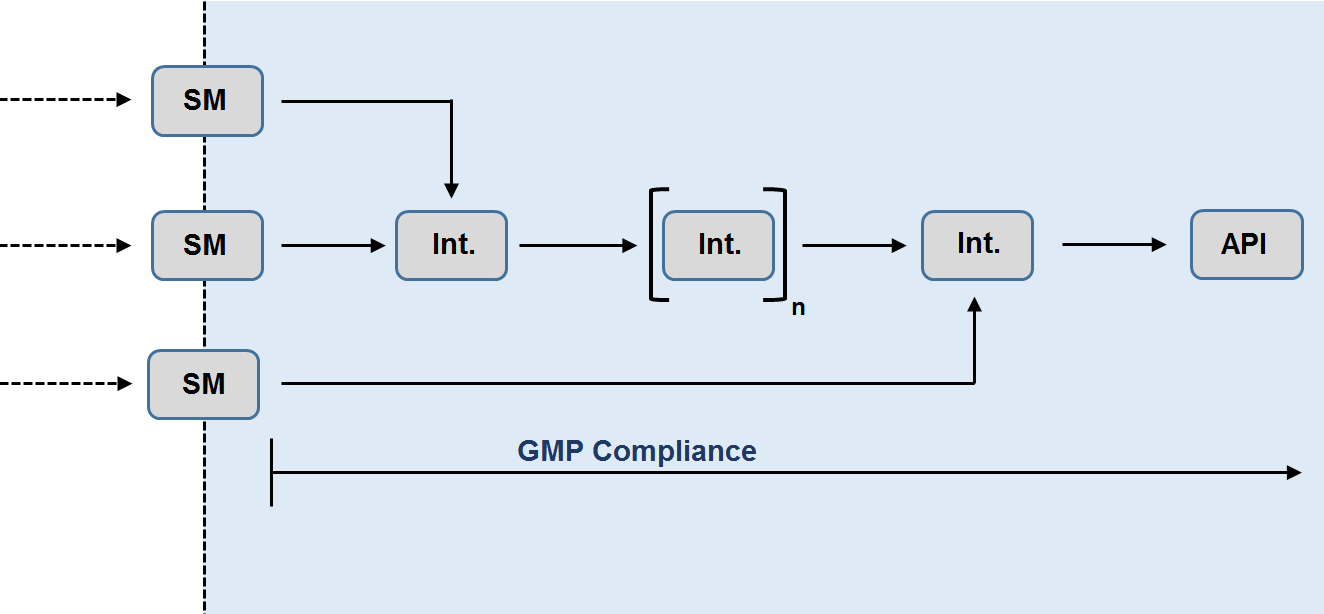
Synthetic scheme:
The synthetic scheme, from the introduction of the starting material(s), should be provided..
- The molecular formula, relative molecular mass and structural formula, including stereochemical configuration, of starting materials, intermediates and the API should be indicated.
- Non-isolated intermediates should be depicted between brackets.
- Solvents, reagents, catalysts and other raw materials used in the manufacturing process should be described and the steps at which they are used should be indicated.
Sequential procedural narrative:
A sequential procedural narrative should be provided, including:
- Process parameters, including quantities or ranges of raw materials, starting materials, intermediates, solvents, catalysts and reagents used in the manufacture of industrial-scale batches, as well as operating conditions (e.g., temperature, pressure, pH, time, flux, etc.), should be provided;
- Identification of critical steps and process controls; and
- Scale of manufacture and yield ranges for each manufacturing step.
Flowchart:
A flow chart of the manufacturing process, containing the sequence of unit operations and indication of input and output of materials and process controls, should be provided.
Reprocessing:
If routine reprocessing is performed, the procedure and circumstances in which it is employed should be described.
Recovered solvents and materials:
If solvents or other recovered materials are used, the maximum ratio and steps from which the solvent/material is recovered and reintroduced should be informed.
Alternative processes:
Alternative processes with substantially differente synthetic routes should constitute separate DIFAs, even if the specifications and impurity profile of downstream intermediates and the API are the same.
Reuse of mother liquors:
If recycled mother liquors are employed, the information should be described in the sequential procedural narrative.
Reworking:
Reworking procedures shoud not be included in the DIFA.
Sterile APIs:
For sterile APIs, the description of the sterilization process should be provided.
API obtained through fermentation:
For APIs obtained through fermentation or in which the substance isolated from the fermentation process or a downstream intermediate does not meet the criteria for selection of starting materials for synthetic APIs, the following additional information should be provided:
- Description of the manufacturing process;;
- Source and type of microorganism;
- Procedures and controls for preparation of master and working cell banks;
- Composition of media;
- control of microbial bioburden in the fermentation process;
- Precursors or metabolic substrates if applicable;
- process parameters (time, temperature, rate of aeration, etc.);
- Name and composition of preservatives; and
- Potential presence of adventitious agents based on the type of microorganism used (e.g. mycotoxins, enzymes).
API derived from botanic material:
For APIs derived from botanical materials in which the substance isolated from the botanical material or a subsequent intermediate does not meet the criteria for selection of starting materials for synthetic APIs, the following additional information should be provided:
- Description of the botanical species and the part of plant used for extraction;
- Geographical origin;;
- Time of harvest, if relevant;
- Information on the use of chemical fertilizers, pestices, fungicides, etc.;
- Potential sources of contamination; and
- Process controls and operating conditions.
