CADIFA GUIDANCE FOR ADMINISTRATIVE PROCEDURES Nº 01 (1st Version)
 (pdf)
(pdf)
TABLE OF CONTENTS
4.1 Module 1: Administrative Information
4.2 Module 2: Quality Overall Summary
ANNEX 1: HOW TO ACCESS SUBMISSION SYSTEM
ANNEX 2: INITIAL SUBMISSION (NON-ELECTRONIC SUBMISSION)
ANNEX 3: INITIAL SUBMISSION (ELECTRONIC SUBMISSION)
ANNEX 4: CHANGE AND OTHER APPLICATIONS (NON-ELECTRONIC SUBMISSION)
ANNEX 5: CHANGE AND OTHER APPLICATIONS (ELECTRONIC SUBMISSION)
ANNEX 6: RESUME OF UNCOMPLETED SUBMISSION
ANNEX 7: REPRINTING SUBMISSION RECEIPT
ANNEX 8: HOW TO ACCESS SOLICITA
ANNEX 9: RESPONSE APPLICATIONS
ANNEX 10: ACCESSING SOLICITA MAILBOX AND READING MESSAGE
I. INTRODUCTION
CADIFA (letter of suitability of the active pharmaceutical ingredient) is the administrative instrument that attests the compliance of a DIFA (active pharmaceutical ingredient dossier) with the requirements of Resolution – RDC nº 359/2020. In other words, it attests that the quality of the API (Active pharmaceutical ingredient) is suitably controlled by the tests that comprise its specification. A CADIFA does not replace a certificate of analysis nor does it ensure that a batch of the drug substance or active pharmaceutical ingredient is of suitable quality.
Although the manufacturer must ensure that the API is produced according to GMP (good manufacturing practice) requirements, the assessment of a CADIFA application does not have the purpose of verifying GMP compliance. Therefore, a CADIFA is neither equivalent to a GMP certificate nor does it replace it.
A valid CADIFA and GMP certificate are necessary for the approval of an associated marketing authorization or post-approval change application. In this document, marketing authorization or post-approval change, when mentioned, are always in reference to Resolution – RDC nº 200/2017 and Resolution – RDC nº 73/2016. The marketing authorization holder (MAH) is responsible for the API quality used in the manufacture of the drug product.
In order to apply for a CADIFA, a DIFA must be submitted to Anvisa by the DIFA holder. A CADIFA application must be previous and associated to a marketing authorization or post-approval change application. In order to have an associated CADIFA, the DIFA holder must provide either (1) a letter to the MAH, with the DIFA’s reference number, authorizing the use of the DIFA as part of marketing authorization or post approval change application; or (2) a copy of the CADIFA with the declaration access filled on behalf of the MAH by DIFA holder, when a CADIFA has already been issued for that API.
Additionally, at its discretion, Anvisa may request standalone CADIFA applications after the approval of an Expression of Interest or through a public invitation Dicol (Anvisa Board of Directors).
Due to the absence of legal provision, applications related to CADIFA are exempted from Health Regulatory Fee (TFVS) paying.
The DIFA’s structure and content have been proposed based on the CTD (Common Technical Document), in ICH M4(R4) and ICH M4Q(R1). This document provides additional guidance on how to organize and submit the DIFA and related documents to Anvisa. Also, it addresses the communication between Anvisa and the DIFA holder.
Information and requirements described in this document are intended to facilitate the handling and assessment of submissions related to the CADIFA. Applicants can adjust the format to improve the presentation and facilitate the understanding and evaluation of the results.
The submission can be either electronic or non-electronic. Anvisa encourages CADIFA applications through electronic submission. If any issue is not addressed in this document, the DIFA holder should contact ANVISA through api@anvisa.gov.br. Please refer the subject [CADIFA Guidance].
II. SCOPE
This document should be used for all CADIFA-related applications, as well as for communications between Anvisa and the DIFA holder related to applications.
III. BEFORE SUBMISSION
CADIFA-related applications and communications will be through systems provided by Anvisa. They are the interface between the DIFA holder and Anvisa to exchange regulatory information. Through it, DIFA holder will submit information to and receive information from Anvisa in a secure platform.
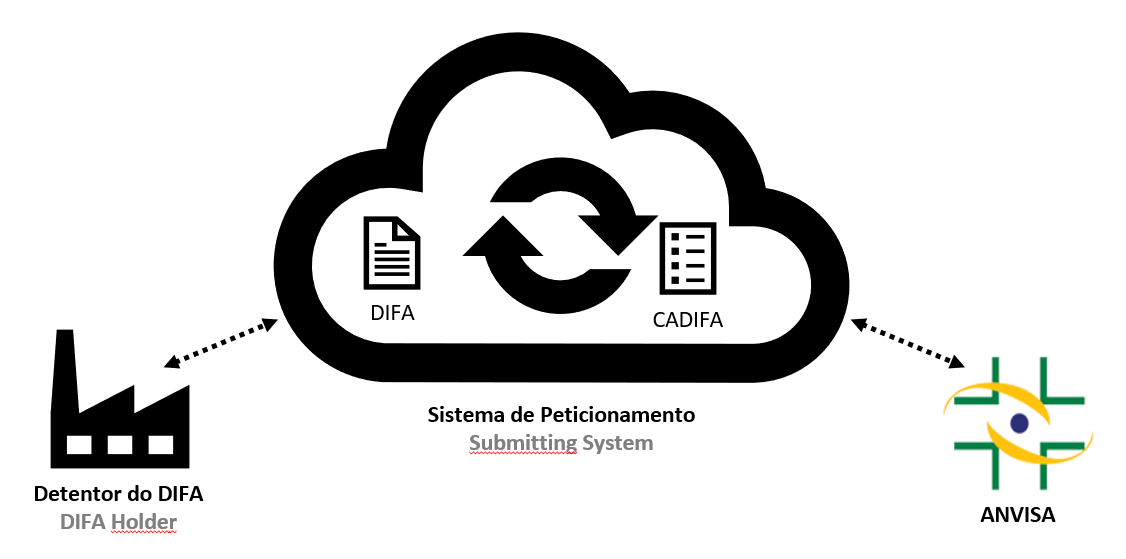
Before accessing Anvisa’s systems, the DIFA holder must first register, choose an application type and organize the documentation.
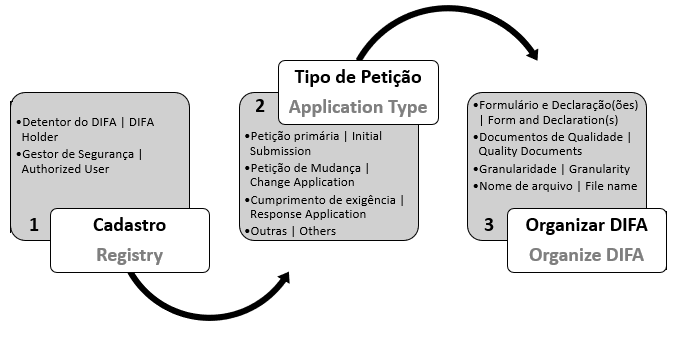
1. REGISTRY
Registry is the procedure through which the DIFA holder is granted access to Anvisa’s systems. In this step, DIFA holder must indicate its authorized user.
According to Resolution – RDC nº 359/2020, DIFA holder is a “company that possesses the knowledge about the entire manufacturing process of the API and under whose oversight the manufacture of the API is conducted, from the introduction of the starting material.”. Preferably, the DIFA holder should be the manufacturer of API. In special cases, the manufacturer(s) may be legally represented by an Authorized Company who will be the DIFA holder. Submission by third parties is not accepted.
The Authorized User will act on behalf of the DIFA holder in Anvisa’s systems. They must be nominated by the DIFA Holder Representative and will have access to all regulatory information submitted by themselves and to all regulatory information sent from ANVISA, including confidential information. There is no limit number of Authorized Users, Anvisa recommends at least two.
How to register?
The registration procedure will depend on whether the DIFA holder (or Representative Company) is legally established in Brazil (i.e., has a CNPJ (Brazilian Corporate Taxpayer Registration) number)..
- With CNPJ number, please proceed to Cadastramento de Empresa (hereinafter Registry System); or
- With no CNPJ number, please fill the Registry Form, sign and send it to difa.holder@anvisa.gov.br with the subject [Registry]. After registration, a DIFA Holder Number (DHN) and Authorized User Number (AUN) will be given.
It is extremely important that information regarding authorized user be updated. Anvisa communicates only with the authorized user. In addition, failure to acknowledge a communication from Anvisa may lead to a rejection of an application.
A registry change must follow the same above procedure, regardless of the need for a DIFA change application (administrative changes in Resolution – RDC nº 359/2020 Annex II)..
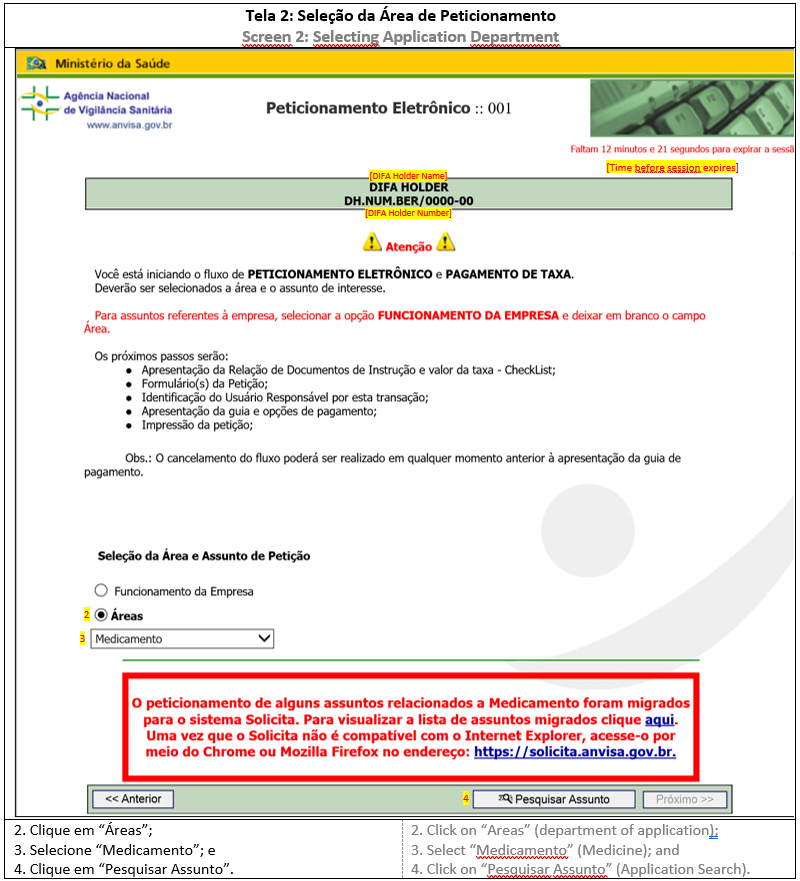
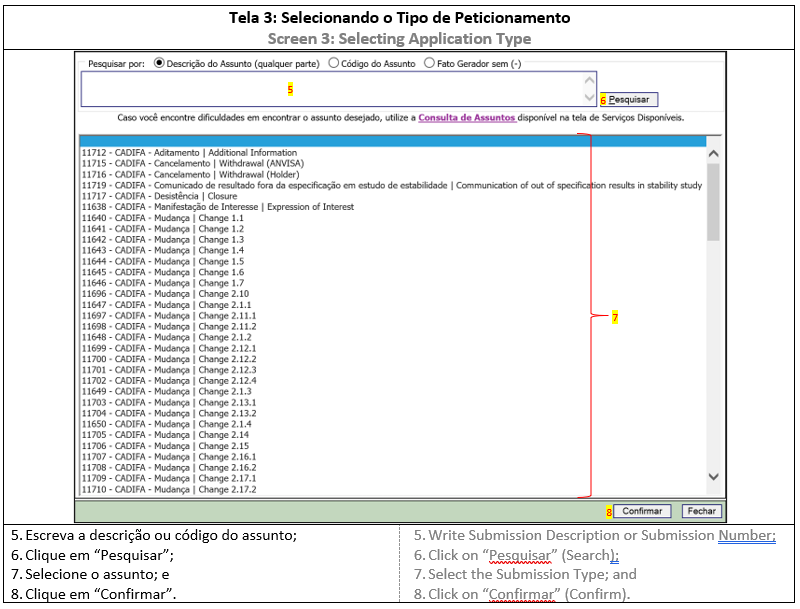
2. APPLICATION TYPE
Before accessing the Submission System, the DIFA holder should choose the application type in List of CADIFA Related Applications, which will determine the submission steps and documents to be uploaded. All applications related to CADIFA belong to “Medicine” department and have a code, description and checklist., já que ele determina o desenvolvimento da solicitação e os documentos que deverão ser apensados. Todas as petições são da área Medicamento e possuem um código, uma descrição de assunto e uma lista de verificação.
All submissions will be given an Application Number. Additionally, the initial submission will have a Reference Number (DIFA reference number).
2.1 Initial Submission
The Initial Submission can be submitted through either a CADIFA Application, Expression of Interest or Standalone CADIFA Application. It will be given a reference number.
- An Associated CADIFA Application must be submitted when a CADIFA is associated with a Marketing Authorization or post approval change application. In this case, the reference number must be indicated in the marketing authorization or post approval change application. If not, the Associated CADIFA Application will not be assessed and, then, closed;
- An Expression of Interest should be submitted when a DIFA holder intends to have a CADIFA through a standalone procedure (i.e., not yet associated to a marketing authorization or post approval change application). Only the Application Form will be submitted in this moment; and
- A Standalone CADIFA Application must be submitted when demanded by Anvisa either after an Expression of Interest approval or a public invitation issued by Anvisa’s Board of Directors (Dicol).
In this Guidance, when CADIFA Application is mentioned, it will refer to an Associated CADIFA Application and Standalone CADIFA Application.
| Application Type | When to apply | |
|---|---|---|
| 1 | Associated CADIFA Application | CADIFA associated with a marketing authorization or post approval change application |
| 2 | Expression of Interest | No marketing authorization or post approval change application demanding CADIFA |
| 3 | Standalone CADIFA Application | After Expression of Interest approval or public invitation issued by Anvisa’s Board of Directors (Dicol) |
Similar DIFA: A DIFA holder may wish to apply for another CADIFA for the same API, either when it is not possible to apply for a change or when the holder wishes to have separate CADIFAs for different conditions of preparation or qualities (for example, to cover an alternative manufacturing process, manufacturing site or an alternative grade).
In order to allow applicants benefit from a faster and harmonized assessment, this similar DIFA can reference another DIFA (approved or waiting for assessment). See how to evidence it in the Submission Form.
It is expected some conditions to be fulfilled for both applications:
- The DCB name and number and CAS number are the same;
- The API is obtained by the same means (extraction from botanical material, synthesis, classic fermentation or semisynthesis;
- The DIFA holder is the same (or belongs to the same group); and
- Manufacturers are the same.
2.2 Change Application
After the CADIFA is granted and as part of approved DIFA lifecycle management, the DIFA holder must submit a Change Application, linked to CADIFA’s reference number, according to conditions and supporting documentation described in Resolution – RDC nº 359/2020 Annex II.
Change codes are available in List of CADIFA Related Applications. The maintenance of DIFA’s lifecycle management is the primary condition for CADIFA remains valid. THE CADIFA DOES NOT HAVE A RENEWAL PROCEDURE.
Any change not classified in Resolution – RDC nº 359/2020 Annex II is classified as minor and should be submitted as “Minor by Default” application type.
Grouped Changes:
Associated changes or those arising from others should be grouped. In this case, the stricter reporting category should be used classification and implementation.
Stricter Category

|
Change Type | Implementation Type |
| Annual Notification | Immediate Implementation | |
| Immediate Notification | Immediate Implementation | |
| Minor | After approval* | |
| Minor by Default | After approval** | |
| Major | After approval** | |
|
*Or after 60 days without Anvisa’s communication.
**Or after 180 days without Anvisa’s communication. |
||
If more than one change is classified in the stricter category, Anvisa suggests choosing, in this order of preference, the change application evolving: (1) API quality control, (2) container closure system/storage condition/shelf life or retest period; (3) manufacture and (4) change management protocol/design space.
A single decision will be issued for grouped changes. If the DIFA holder would like to implement each change separately (because they are not arising one from each other), they may be submitted separately. For instance, an annual or immediate notification submitted together with a minor or major may be implemented only after Anvisa’s final decision or elapsed time for Anvisa’s assessment.
Anvisa encourages annual notifications submission to be compiled with other annual notification which have been implemented within 12 months of the first change. The reporting of annual notifications is based on the implementation date of the changes and not the anniversary of the CADIFA application. When one of these changes determines the CADIFA revision (e.g. 2.7.3 and 2.8.3 changes), it must be chosen to be applied for the submission of all grouped changes.
2.3 Response
During the assessment of a CADIFA Application or a Change, Anvisa may send a Deficiency Letter in order to clarify or require further information. A Response must be submitted in 120 (one hundred and twenty) days by DIFA holder after the acknowledgement of the Deficiency Letter.
2.4 Other Applications
Except from a response application, after any other submission, DIFA holder can submit Additional Information to complement information already submitted. This application cannot be used to send missing mandatory information or change the submission request. This application will be assessed along with the main one (linked during the Additional Information submission).
Additionally, it is possible to request Closure for any submission, while Anvisa has not made a final decision.
DIFA holder may request CADIFA Suspension (limited to a period of 2 years) or Withdrawal.
Reconsideration can be submitted after a rejection of any submission. In this case, due to legal restrictions, it is only accepted when submitted by a Brazilian Authorized User. Additionally, if a MA or post-approval change application is partially or fully rejected due to information pertaining to the DIFA, the MAH can submit a Reconsideration in its associated Marketing Authorization Application and, if applicable, refer to documentation provided by DIFA holder in its DIFA process.
3. SUBMISSION FORMAT
Anvisa provides two types of submission format (electronic and non-electronic) in the Submitting System for applications related to CADIFA. The subsequent submissions must follow the same format of the initial submission, except for applications which are only available in electronic protocol.
The Non-Electronic Submission must be in CTD format, provided on media (USB flash drive) and follow the submission requirements in “PROTOCOL” section of Guia 24/2019, versão 1, translated below. For this format, the regulatory transaction will initiate in the Submitting System and conclude when the media is delivered at Anvisa headquarters (either in person or through mail).
Guia nº 24, versão 1, de 14 de agosto de 2019, “PROTOCOL” section.
Translation of “PROTOCOL” section adapted for, when available, Non-Electronic Submission of applications related to CADIFA.
PROTOCOL
[...]
All files must comply with the requirements in “GENERAL PRINCIPLES” [ICH M4(R4)] and the following:
I – PDF (portable document format), allowing textual search and copy;
II – up to 60MB (megabytes) per file.
The media must be on an USB flashdrive delivered in a package labeled with: Company Name, API and reference number.
[...]
The following applications will not be received:
I – do not follow this procedure requirements;
II – the media is damaged or with corrupted files;
III – the media do not have any content;
IV – the media or file is password protected.
[...]
All applications must have a Cover Letter [see how to fill it here] application form and TFVS payment receipt [Due to the absence of legal provision, applications related to CADIFA are exempted from Health Regulatory Fee (TFVS) paying]. These are the only documents that must be printed and sent along with the packaged media. Additionally, the cover letter should be provided at 1.2.1 folder, the application form at 1.2.3 folder and taxpayment receipt at 1.2.5 folder of Module 1 on media.
[...]
The Electronic Submission must follow the requirements in this guidance and will be initiated and concluded electronically, with the files uploaded to the Submission System. A submission receipt will be issued.
4. ORGANIZATION OF DIFA
A DIFA contains three modules: Module 1 (Administrative Information) , Module 2 (Common Technical Document Summaries) and Module 3 (Quality). For electronic submission, the Module 2 is optional.
Anvisa encourages the adoption of “General Principles” “Annex: Granularity Document”, “Document Pagination and Segregation”, “Section Numbering within Documents”, “Table of Contents Formatting” according to ICH M4(R4).
File Requirements: Files must be submitted in PDF (portable document format), allowing textual search and copy, and no larger than 60 MB (megabytes) for non-electronic submission and 20 MB for electronic submission. Larger files should be split into smaller components. Anvisa encourages the use of PDF documents produced from an electronic source document, those produced by scanning paper documents are usually inferior to those produced. Scanned documents saved as image files are more difficult to read and do not allow reviewers to search or copy and paste text for editing. Scanning should be avoided whenever possible. No security settings or password protection for PDF files should be included. Security fields should be set to allow printing, changes to the document, selecting text and graphics, and adding or changing notes and form fields.
File Naming: Anvisa recommends file names presented here. These file names are not mandatory, but recommended, and can be further complemented, reduced or omitted. In some sections, it can be helpful if the file name includes the name of the validated analytical procedure. The subsequent submissions should use the same file names of the initial submission.
4.1. Module 1: Administrative Information
In Module 1, administrative documents required at Resolution – RDC nº 359/2020 according to application type must be submitted to Anvisa. The format and order of Module 1 is described in Annex I of Guia 24/2019, versão 1, adapted and translated below.
Structure of Module 1, according to Annex I of Guia 24/2019, versão 1.
1.1 Summary
In this section, a summary for all CTD modules should be provided, including page number of each document. This section is mandatory only for non-electronic submissions.1.2 Administrative Information
1.2.1 Cover Letter
This section is mandatory only for non-electronic submissions which must have a Cover Letter, (see how to fill it here).
1.2.2 Justification
A brief justification for the application and for the absence of any required document/information. This information can be also stated in the application form’s field 1.1.2.
1.2.3 Application Form
The Application Form must be submitted in this section.
1.2.4 Response
In a Response application, a letter answering each question of the deficiency letter and correlating the new DIFA sections of Module 3 should be submitted, if updated.
1.2.5 TFVS Payment Receipt
Due to the absence of legal provision, applications related to CADIFA are exempted from Health Regulatory Fee (TFVS) paying.
[...]
1.3 Communication with Anvisa
1.3.1 Minutes of Meeting
Unless otherwise requested, it is not mandatory. In this section, minutes of any meeting with Anvisa related to the application can be provided.
1.3.2 Anvisa’s answers by e-mail due to a DIFA Holder’s question
Unless otherwise requested, it is not mandatory. In this section, any answers provided by Anvisa to questions raised by the DIFA holder related to the application can be provided.
1.3.3 Anvisa’s letter due to a DIFA Holder’s question
Unless otherwise requested, it is not mandatory. In this section, any answers provided by Anvisa to questions raised by the DIFA holder related to the application can be provided.
[...]
1.4 Further Administrative Information
Unless otherwise requested, it is not mandatory. In this section, any other administrative information, not provided in the previous sections, can be provided.[...]
1.6 Information of API
[...]
1.6.4 International Regulatory Information
Unless otherwise requested, it is not mandatory. In this section, if applicable, copy of the Certificate of Suitability (CEP) issued by European Directorate for the Quality of Medicines & Healthcare (EDQM) or information that the API is prequalified by WHO (World Health Organization).
1.7 Change, Response and Other Applications
[...]
1.7.4 Supporting Documentation
The supporting documentation required at Annex II of Resolution – RDC nº 359/2020, according to the change(s) applied.
[...]
The Module 1 documents must be submitted in PDF. Anvisa provides templates of a cover letter, (see how to fill it here), application form and declarations, which must be signed. The application form template is structured for all types of applications. Additionally, Anvisa suggests the submission of the Comparative table, when required, in DOC/DOCX format.
Subtitle: In the 1.2.1 field of the Application Form, the DIFA holder may request that a subtitle name to be included along with the INN API name on the CADIFA (e.g. particle size, polymorphic form, sterile, etc.). Also, it important to differentiate a Similar DIFA from the original DIFA. For instance, it can represent the physical characteristic of the substance (polymorphic form II, etc.) or an alternative process (process 2, site X, code number, etc.).
Similar DIFA: Anvisa suggests filling the Application Form mentioning this situation it in 1.1.2 field, the original DIFA in 1.1.3 field and a subtitle in 1.2.1 field. The differences between DIFAs in Comparative Table (Application Form Annex 9) is a key document determining how fast the assessment will be.
Response: Unless otherwise required, it is NOT necessary to submit an application form when responding to a deficiency letter. However, applicants should include a letter (provided at section 1.2.4 of Module 1) listing the questions with the corresponding responses and supportive data, and, when applicable, making cross reference to updated sections of the dossier.
At the discretion of DIFA holder, other administrative documents can be submitted, following the Module 1 structure. Summary of mandatory, optional and not applicable administrative documents to be submitted, depending on protocol and application type.
| Module 1 | Applications | ||||||
|---|---|---|---|---|---|---|---|
| Section | Initial Submission | Change | Response* | Others* | |||
| 1.1 | Table of Contents | (M) | O | ||||
| 1.2 | Administrative Information | 1.2.1 | Cover Letter | (M) | NA | ||
| 1.2.2 | Justification | O | NA | O | |||
| 1.2.3 | Application Form | (M)(E) | NA | (E) | |||
| 1.2.4 | Response | NA | (E) | NA | |||
| 1.2.5 | TFVS Payment Receipt | (M) | NA | ||||
| 1.2.6 | NA | ||||||
| 1.2.7 | |||||||
| 1.2.8 | |||||||
| 1.3 | Communication with Anvisa | 1.3.1 | Minutes of Meeting | O | |||
| 1.3.2 | Anvisa’s answers by e-mail due to a DIFA Holder’s question | ||||||
| 1.3.3 | Anvisa’s letter due to a DIFA Holder’s question | ||||||
| 1.3.4 | NA | ||||||
| 1.3.5 | |||||||
| 1.4 | Further Administrative Information | O | |||||
| 1.5 | NA | ||||||
| 1.6 | Information of API | 1.6.1 | NA | ||||
| 1.6.2 | |||||||
| 1.6.3 | |||||||
| 1.6.4 | |||||||
| 1.6.5 | International Regulatory Information | O | |||||
| 1.7 | Change, Response and Other Applications | 1.7.1 | NA | ||||
| 1.7.2 | |||||||
| 1.7.3 | |||||||
| 1.7.4 | Supporting Documentation | NA | (M)(E) | O | |||
| 1.7.5 | NA | ||||||
|
* These applications are available only for electronic protocol.
When to submit? (M) – Mandatory only for Non-Electronic Submission; (E) – Mandatory only for Electronic Submission; (M) (E) – Mandatory for both types of submission format; O – Optional for both types of submission format; and NA – Not applicable for both types of protocol. |
|||||||
4.2. Module 2: Quality Overall Summary
The Module 2 is the quality overall summary which follows the scope and structure of Module 3. The format, organization and granularity must follow the ICH M4Q(R1). Module 2 submission is mandatory for non-electronic submissions.
| Module 2 | Applications | ||||||
| Section | Initial Submission | Change | Response | Others | |||
| 2.1 | Table of Contents (Modules 2-3) | (M) | NA | ||||
| 2.2 | Introduction | O | |||||
| 2.3 | Quality Overall Summary | 2.3.S | Active Pharmaceutical Ingredient | (M) | |||
|
When to submit?
Mandatory only for Non-Electronic Submission; Optional for both types of protocol; and Not applicable for both types of protocol. |
|||||||
4.3 Module 3: Quality
Module 3 contains the documents with quality information, as required in Resolution – RDC nº 359/2020. The format, organization and granularity must follow ICH M4Q(R1).
When there are confidentiality restrictions between DIFA holder and the MAH, the information on quality should be divided into two parts, namely the Applicant’s Part (AP) and the Restricted Part (RP), according to Annex III of Resolution – RDC nº 359/2020. The AP should contain sufficient information to enable the Applicant/MA holder to take full responsibility for an evaluation of the suitability of the specification for the API to control the quality of this API for use in the manufacture of a specified medicinal product.
IV. SUBMISSION SYSTEMS
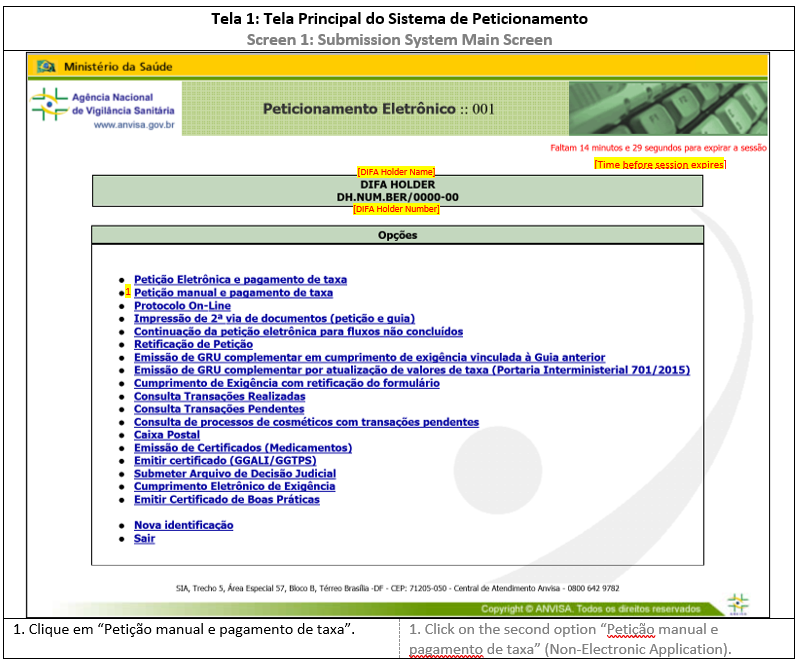
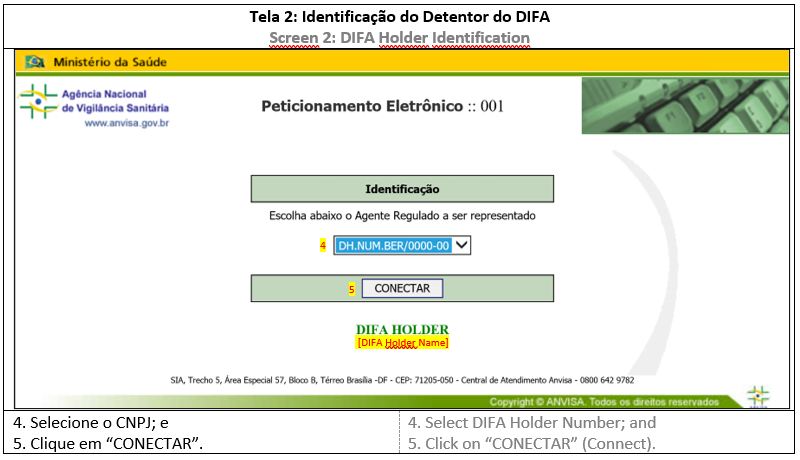
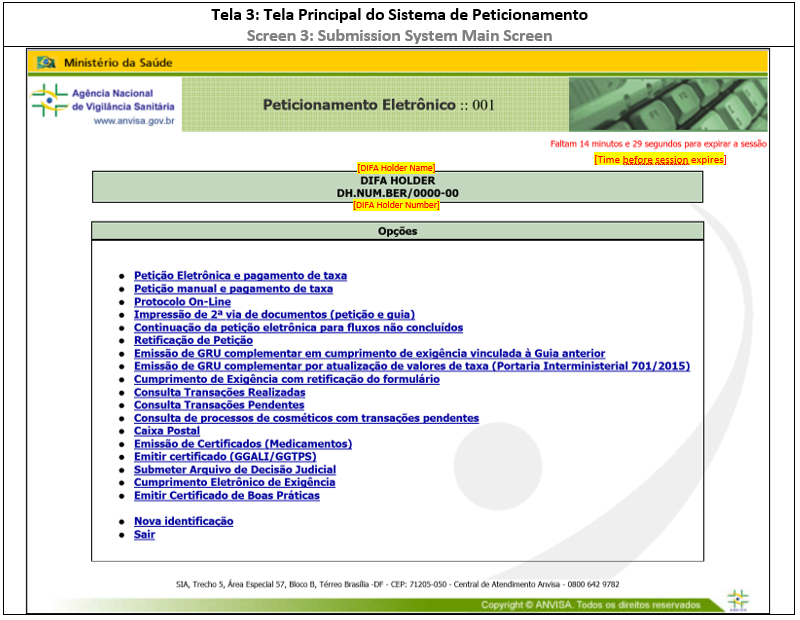
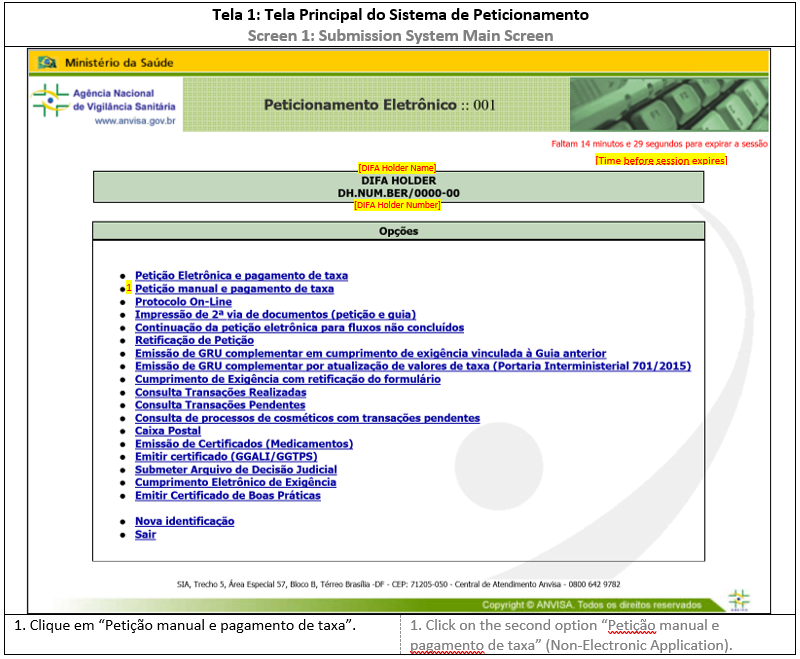
Except for the response application, the submission of documents related to CADIFA applications will be submitted through Peticionamento Eletrônico. To access the system, see ANNEX 1: HOW TO ACCESS SUBMISSION SYSTEM. In the main screen, it is possible to proceed with further actions.
If you see the below message error appear, you must add Anvisa’s website in your “Compatibility View” to solve this problem. After accessing Submission System’s website, please go to “Tools” in your IE11 and click on “Compatibility View settings”. Then click on “Add”, to add “anvisa.gov.br” website in the compatibility mode.

The Solicita system must be used for:
- Accessing communications issued by Anvisa (i.e., deficiency letters, other letters and the CADIFA); and
- Submitting of responses to deficiency letters.
| Submission Systems | ||
| Peticionamento Eletrônico | Sistema Solicita | |
| Procedure | Application | Response and Mailbox |
| User login and password | Authorized User | |
| Browser | Internet Explorer 11 | Chrome or Mozilla Firefox |
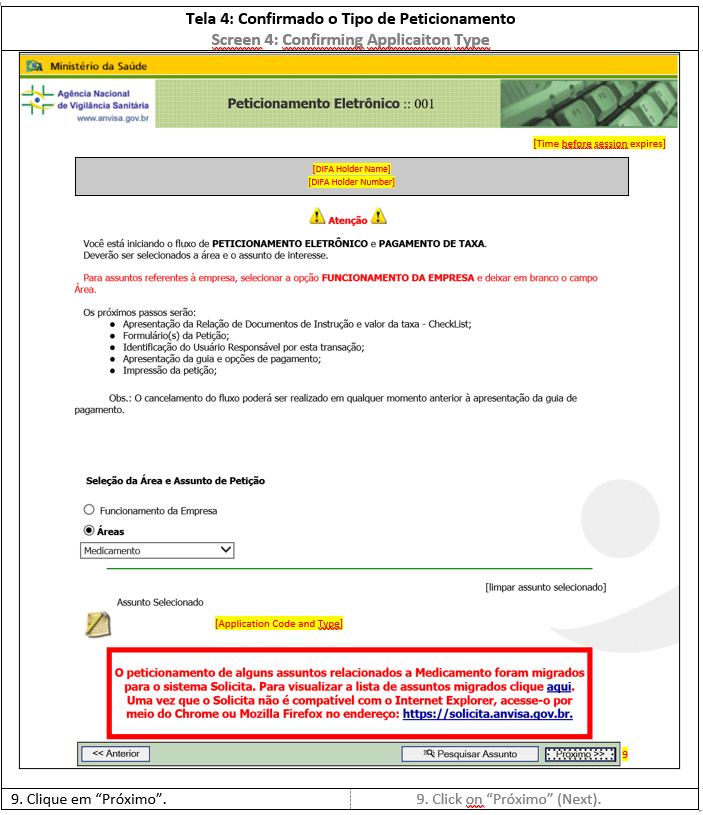
Transaction Number: Regardless the application type, after application type confirmation, a transaction number will be generated and immediately sent to the authorized user from Anvisa (sistemas@anvisa.gov.br). After application submission conclusion, this message is also sent to the company’s e-mail and, again, to the authorized user’s e-mail.

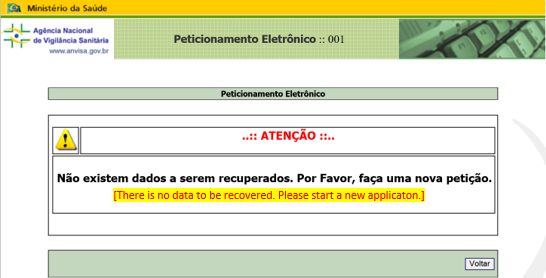
The transaction number allows the submission procedure to be resumed from “Application Type Details” screen. If the session expires, see ANNEX 6: RESUME OF UNCOMPLETED SUBMISSION. If the below message (Figure 4) appears after procedure, a new application should be started.
Checklist: Every application type has a specific checklist with documents which must be uploaded during the submission. After the upload, each document must be visualized before the authorized user goes to the next submission step.
1. NON-ELECTRONIC SUBMISSION
For this submission format, the regulatory transaction will initiate in the Submission System and conclude after receiving the documents and media (USB flash drive) at Anvisa headquarters, when a reference number and application number is given.
After the procedure in the Submission System, only a transaction receipt and an exemption of TFVS will be issued. These documents must be printed and posted to Anvisa’s headquarter together with:
- Cover letter (printed);
- Application form (printed); and
- Media (USB flashdrive) in a sealed package identified with Company name, API and transaction number (or the reference number, for subsequent submissions).
|
À Agência Nacional de Vigilância Sanitária (Anvisa)
COIFA SIA Trecho 5, Área especial 57, Lote 200 CEP: 71205-050 Brasília – DF, Brasil |
For CADIFA Application or Expression of Interest, see ANNEX 2: INITIAL SUBMISSION (NON-ELECTRONIC SUBMISSION). Special attention must be given to the chosen granularity, which will determine the file upload during the submission. This granularity should be the same in the following applications.
For subsequent submissions (other than a response application), as Change or Others, see ANNEX 4: CHANGE AND OTHER APPLICATIONS (NON-ELECTRONIC SUBMISSION). The association of this application to DIFA’s reference number will be made by Anvisa, after receiving the documents and concluding the regulatory transaction.
2. ELECTRONIC SUBMISSION
The application by electronic submission must follow the instructions in this Guidance. It will be initiated and concluded in the Submission System, with a receipt issued.
Submission Receipt: After any submission by electronic submission, a receipt will be issued with the application type, authorized user and DIFA holder information. When a CADIFA Application is submitted, it is important to forward the Reference Number to the MAH in its submission.

If necessary, the receipt can be reprinted, as in ANNEX 7: REPRINTING SUBMISSION RECEIPT ON-LINE.
For CADIFA Application or Expression of Interest, see ANNEX 3: INITIAL SUBMISSION (ELECTRONIC SUBMISSION). Special attention must be given to the chosen granularity, which will determine the file upload during the submission. This granularity should be the same in the following applications.
For subsequent submissions (other than a response application), as Change or Others, see ANNEX 5: CHANGE AND OTHER APPLICATIONS (ELECTRONIC SUBMISSION).
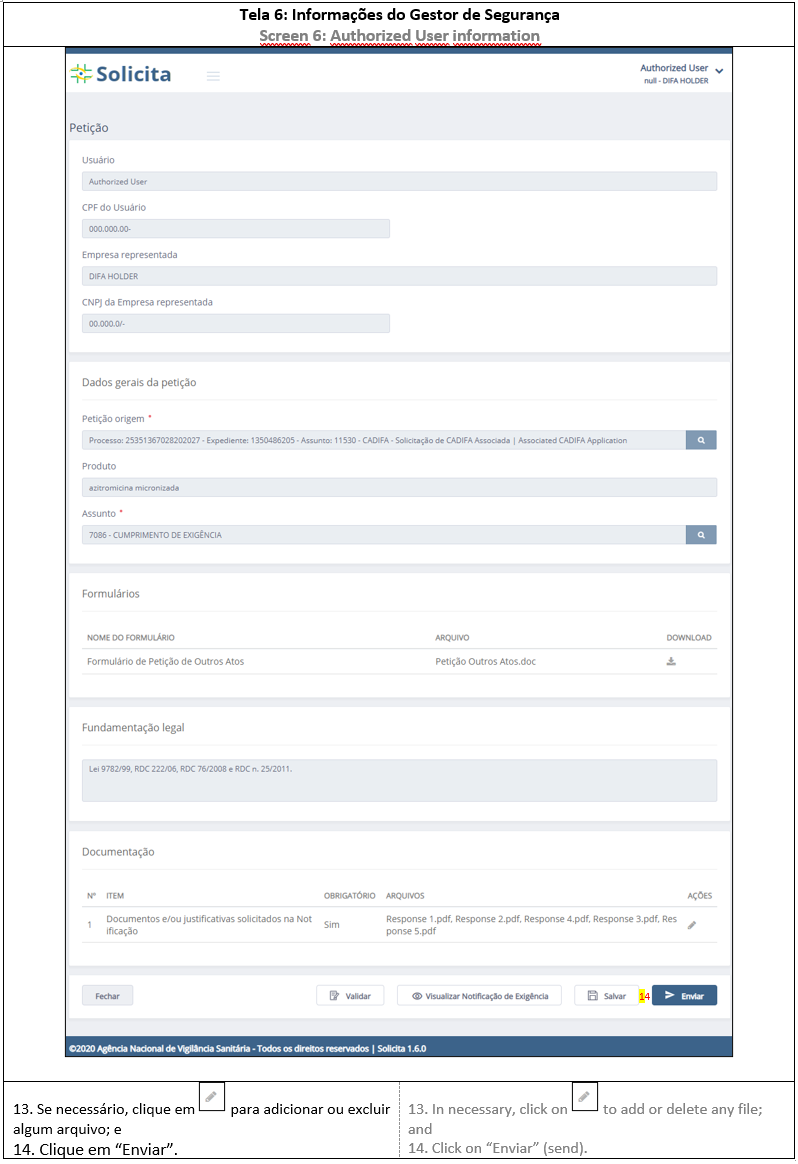
3. RESPONSE
For a Response application, the deficiency letter number will be necessary, see how apply in ANNEX 9: RESPONSE APPLICATION.
4. MAILBOX
After any application or communication by Anvisa , when accessing the Solicita system, the Authorized User will be automatically notified of unread messages in the mailbox. Only after reading all unread message(s), it will be possible to submit a new application.
To access the Mailbox in Solicita system and reading a message, see ANNEX 10: ACCESSING SOLICITA MAILBOX AND READING MESSAGE.
Anvisa will not send notifications about new document/message in the Mailbox directly to the authorized user e-mail, Therefore, it is important to monitor the mailbox to answer requests or receive documents from Anvisa.
V. AFTER SUBMISSION
The application status can be consulted at Consulta à Situação de Documentos (Consulting System). See ANNEX 11: APPLICATION STATUS CONSULTING. It is possible to registry an e-mail for application status notifications, weekly or monthly, see ANNEX 12: MONITORING APPLICATION STATUS.
After a CADIFA or Change Application or a Response submission to a Deficiency Letter, ANVISA will assess its compliance to Resolution – RDC nº 359/2020.
| Application | Outcome of Assessment | Decision Communication |
|---|---|---|
|
DIFA
(CADIFA or Change Application) |
||
| Approval |
CADIFA*
/ Approval Letter |
|
| Deficiency Letter | Deficiency Letter Notification | |
| Rejection | Rejection Letter | |
| *new or reviewed | ||
All documents will be sent to DIFA holder’s Mailbox. Anvisa will NOT issue hard copies.
1. APPROVAL
If the DIFA is in accordance of Resolution – RDC nº 359/2020 and guarantees the quality of the API is adequately controlled, a CADIFA is granted or revised or a Letter of Acceptance is issued informing the change request has been accepted. Changes which are accepted will result in revision of the CADIFA only if they are major or determine CADIFA’s information to be updated.
Anvisa will be send the CADIFA only to its holder
2. DEFICIENCY
The submissions are not approved while the information is not satisfactory. In this case, Anvisa may issue a Deficiency Letter to DIFA holder with a list of queries requesting further information or clarification. The DIFA holder has a deadline of 120 (one hundred and twenty) calendar days as of the day of reading to submit a Response. Otherwise, the application will be rejected. Only one round of queries is expected. A second would be exceptional.
3. REJECTION
If the submission is not provided with documentation required by Resolution - RDC nº 359/2020, the submission is rejected in screening. If no Response is submitted, the submission is rejected. If, after one or two rounds of queries, the submission still does not comply with the Resolution - RDC nº 359/2020, the application will be also rejected.
For rejected change application, the current version of CADIFA (without the requested changes) remains valid.
In all cases, ANVISA will send a Rejection Letter with the reasons which prevent the acceptance and have led to rejection. However, the applicant may submit a new application for the same substance or change, it is expected that the outstanding issues from the assessment of the original closed dossier will be addressed in the new submission.
4. CLOSURE
The DIFA holder may request the closure of any application before Anvisa’s final decision. The applications will have its status changed to “desistência a pedido” (closure on request) and a Closure Letter will be sent to DIFA holder.
VI. REFERENCES
- ANVISA. Resolução de Diretoria Colegiada – RDC nº 359, de 27 de março de 2020. 01/04/2020.
- ANVISA. Resolução de Diretoria Colegiada – RDC nº 200, de 26 de dezembro de 2017. 29/01/2018.
- ANVISA. Resolução de Diretoria Colegiada – RDC n° 73, de 7 de abril de 2016. 11/04/2016.
- ANVISA. GUIA nº 24, versão 1. 14/08/2019.
- ICH. ICH M4(R3). 13/01/2004.
- ICH. ICH M4Q(R1). 12/09/2002.
- ANVISA. Resolução de Diretoria Colegiada – RDC nº 222, de 28 de dezembro de 2006. 29/12/2006.
- ANVISA. Resolução de Diretoria Colegiada – RDC nº 204, de 06 de julho de 2005. 07/07/2005.
ANNEX 1: HOW TO ACCESS SUBMISSION SYSTEM
ANNEX 2: INITIAL SUBMISSION (NON-ELECTRONIC SUBMISSION)
ANNEX 3: INITIAL SUBMISSION (ELECTRONIC SUBMISSION)
ANNEX 4: CHANGE AND OTHER APPLICATIONS (NON-ELECTRONIC SUBMISSION)
ANNEX 5: CHANGE AND OTHER APPLICATIONS (ELECTRONIC SUBMISSION)
ANNEX 6: RESUME OF UNCOMPLETED SUBMISSION
ANNEX 7: REPRINTING SUBMISSION RECEIPT ON-LINE
ANNEX 8: HOW TO ACCESS SOLICITA
ANNEX 9: RESPONSE APPLICATIONS
ANNEX 10: ACCESSING SOLICITA MAILBOX AND READING MESSAGE
ANNEX 11: APPLICATION STATUS CONSULTING
ANNEX 12: MONITORING APPLICATION STATUS